
What is the Li-ion battery? Is this battery technology becoming obsolete or will it roll onto the future with new developments? Here we attempt to answer such questions about the Lithium-ion (Li-ion) battery used in our smartphones and other gadgets.
The prevalence of smartphones has pervaded our daily lives. It is commonplace to see at least one, if not two, smartphones in the hands of people, especially youngsters, waiting at railway and bus stations; riding buses, trains; or just plain waiting.
The juice on most of these phones today is provided by the most popular battery option—the Lithium-ion (Li-ion) battery pack. Such is the ubiquity of this battery type that these days, even a normal person can understand jargon terms such as battery capacity (measured in terms of a unit called milliamp hour, abbreviated as mAh). In a nutshell, higher the value of mAh, the longer the battery will last until it requires recharging.
Consumers have started to become more aware of the equipment that they are using. Hence, even the manufacturing companies have begun throwing all kinds of technical mumbo-jumbo at them. Terms like fast charging, turbocharging, dash charging, large battery capacity, etc. are used by companies to lure consumers into buying their products.
Therefore, it has become imperative to understand what a battery is and, on the basis of current trends and research, see where the battery will evolve into in the coming years.
What is the Li-ion battery?
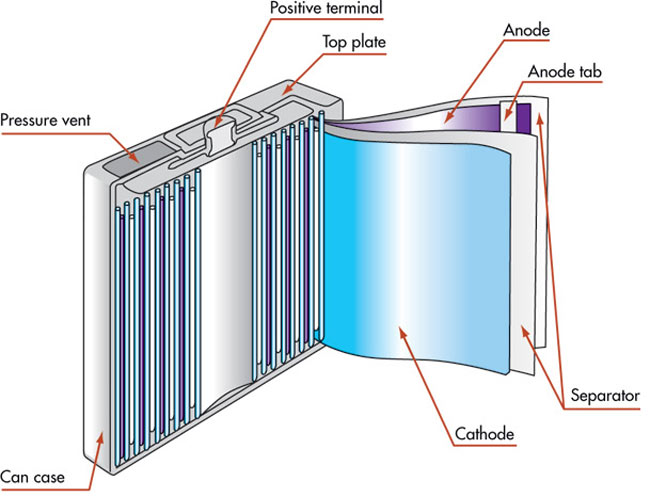
In order to have a slightly better understanding of a battery, let’s take a look inside one:
Electricity cannot be stored per se, but electrical energy can be stored in the form of chemical energy inside a battery. A battery is basically a slow chemical reaction in progress. Depending on the type of battery (rechargeable and non-rechargeable), the chemicals used in the battery change; however, its basic construction remains the same.
A battery comprises three main parts: a metallic anode, a metallic cathode, and an electrolyte solution separating both of them. The electrolyte allows the transfer of electrical charge between the cathode and the anode. Simply put, when the cathode and anode are interconnected via an electric device (say, torch), the anode and cathode undergo chemical reactions that result in the flow of charge between them (electrons move from the anode to the cathode), passing through the connected electric device. This process refers to as the battery is “discharging.” When charging the battery (in the case of rechargeable battery packs), the movement of electrons is reversed.
A Li-ion battery pack works on a similar principle. The name “Lithium-ion” is based on the active materials used in the battery, i.e., Lithium. There are various types of Li-ion batteries available in the market today. They are Lithium cobalt oxide (the most common type), Lithium manganese oxide, Lithium nickel manganese cobalt oxide, Lithium iron phosphate, Lithium nickel cobalt aluminum oxide, and Lithium titanate.
All these batteries are commercially available and are used as per requirements and suitability. As an example, Lithium titanate batteries are the safest Li-ion batteries; therefore, they are used in UPS systems, street lighting, etc., where safety is an imperative requirement On the other hand, Lithium cobalt oxide batteries are cheap to manufacture; therefore, they are widely used in mobile phones, tablets, laptops, computers, etc.
Evolution of Li-ion Battery Technology
The Li-ion battery was conceptualized in the 1970s. At that time, the constituents of the battery were extremely expensive, because of which it could not be practicalized. However, a rapid pace in material development and process understanding, the Li-ion battery has become ubiquitous in powering consumer electronics across the world.
With ever-increasing consumer demand in the form of massive cellphone sales, smart homes and smart cars, and almost every electronic device requiring battery backup, the amount of R&D money being poured into Li-ion development has increased exponentially in the last couple of decades. This has directly led to an improvement in the storage capacities and charging/discharging capabilities of Li-ion batteries all across the board.
Even though structural changes in the Li-ion battery have not been significant over the last few decades, the amount of money being pumped into developmental costs has resulted in steady improvements in the technology.
Battery capacities have increased significantly over the past decade, and the battery size has reduced. Overall, any consumer wants the following two things in the battery: higher capacity and shorter charging times. As this new trend picks up, R&D efforts are being focused in this direction.
Some examples of innovations that have been developed are Qualcomm’s Quick Charge and OnePlus’ Dash Charge technologies. The latest Quick Charge technology (i.e., Quick Charge 4) ostentatiously claims that five minutes of charging using a Quick Charge 4 charger can give you five hours of battery life. Dash Charging already claims a day’s power in half an hour.
The Future of Li-ion Battery
In the race for superstardom, battery technology is not far behind charging technologies. Some of the trends in Li-ion R&D are listed below:
UT Dallas University, Texas: Lithium-air batteries—in which the electrolyte is replaced by air—have almost 10 times the capacity of conventional Li-ion batteries, and they are one-fifth the cost and weight of an equivalent battery pack. This discovery stands to revolutionize the mobile phone industry, which is already reeling under pressures from consumers wanting more in respect of longevity of their smartphones without requiring frequent charging.
UC, Irvine, California: Researchers in UC Irvine have created nanowire-based battery material that has the capability of being recharged thousands of time, making the pain faced in replacing batteries obsolete.
Pohang University of Science and Technology (POSTECH), South Korea: Scientists at POSTECH have successfully developed miniaturized solid oxide fuel cell (SOFC) that can carry a large capacity of charge in a very small package. It has, for the first time ever, combined porous stainless steel with thin-film electrolytes and electrodes of minimal heat capacity. The research team aims to help drones fly for more than an hour and mobile phone batteries to last up to a week without requiring charging.
Prieto Battery (start-up from Colorado State University): Conventional batteries have a two-dimensional structure (electrodes separated by an electrolyte). Prieto has been successful in developing a “three-dimensional” foam battery, where the anode is coated with “foam,” a polymer electrolyte providing a physical barrier, and the cathode applied in the form of dark, inky slurry. This has created batteries that are a couple of inches thick and paper thin. They charge fast, store twice as much energy per unit volume as compared to conventional batteries, and do not overheat (something that Li-ion batteries are infamous for).
Seeing from the above, good days are ahead for battery technology, which is eventually going to benefit the end user in the not-so-distant future.



Join The Discussion: